Table 5.14: Composition and properties of biogas, and its constituents under s.t.p.
conditions (0 °C, 1013 mbar)
(Source: OEKOTOP, compiled from various sources)
Constituents and properties
Volume fraction (%)
Net calorific value (kWh/m³)
Ignition threshold (% in air)
Ignition temperature (°C)
Crit.pressure (bar)
Crit. temp. (°C)
Normal density (g/1)
Gas/air-density ratio
Wobbe index, K (kWh/m³)
Spec. heat, cp (kI/m³ °C)
Flame propagation (cm/s)
CH4
CO2
55-70
9.9
5-1S
650-750
47
-82.5
0.72
0.55
13.4
1.6
43
27-44
-
-
-
75
31.0
1.98
2.5
-
1.6
-
H2 H2S
1
3.0
4-80
585
13
-240
0.09
0.07
-
1.3
47
3
6.3
4-45
-
89
100.0
1.54
1.2
-
1.4
-
60% CH4/
40% CO2
100
6.0
6-12
650-750
75-89
-82.5
1.2
0.83
6.59
1.6
36
65% CH4/
34%
C02/
1% rest
100
6.8
7.7 - 23
650-750
75-89
-82.5
1.15
0.91
7.15
1.6
38
Reduction of the hydrogen-sulfide content (H2S) may be necessary if the biogas is found to contain
an excessive amount, i.e. more than 2%, and is to be used for fueling an engine. Since, however,
most biogas contains less than 1% H2S, desulfurization is normally unnecessary, especially if it is
to be used for operating a stationary engine.
For small-to-midsize systems, desulfurization can be effected by absorption onto ferric hydrate (Fe
(OEI)3), also referred to as bog iron, a porous form of limonite. The porous, granular purifying mass
can be regenerated by exposure to air.
The absorptive capacity of the purifying mass depends on its iron-hydrate content: bog iron
containing 5-10% Fe(OH)3 can absorb about 15 g sulfur per kg without being regenerated and
approximately 150 g/ kg through repetitive regeneration. It is a very noteworthy fact that many types
of tropical soil (laterites) are naturally ferriferous and, hence, suitable for use as purifying mass.
Reduction of the carbon-dioxide content (CO2) is very complicated and expensive. In principle, CO2
can be removed by absorption onto lime milk, but that practice produces "seas" of lime paste and
must therefore be ruled out, particularly in connection with large-scale plants, for which only high-
tech processes like microscreening are worthy of consideration. CO2 "scrubbing" is rarely
advisable, except in order to increase the individual bottling capacity for high-pressure storage.
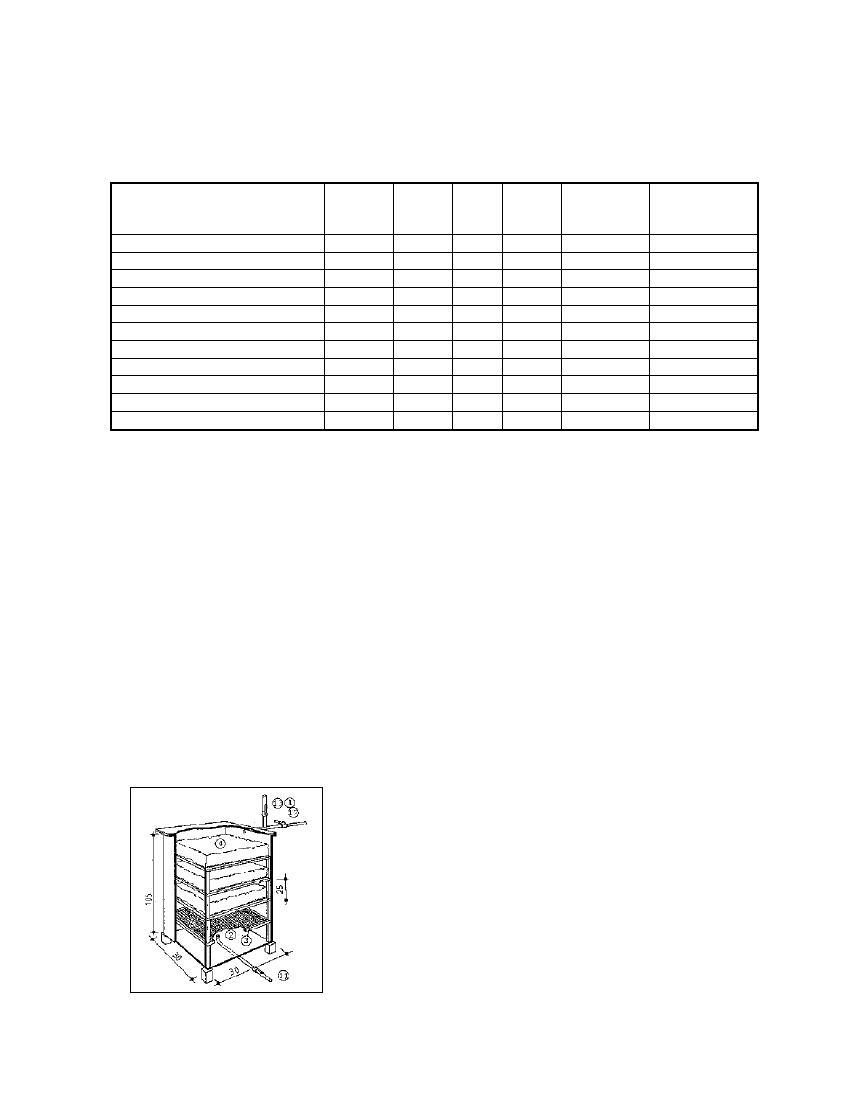
Fig. 5.29: Ferric-hydrate gas purifier. 1 Gas pipe, 11 Raw-gas
feed pipe, 12 Clean-gas discharge pipe, 13 Purging line, 2 Metal
gas purifier, 3 Shelves for purifying mass, 4 Purifying mass
(Source: Muche 1984)
66