Khan Academy on a Stick
Reaction rates
-
 Introduction to kinetics
Introduction to kinetics
Kinetics, activation energy, activated complex and catalysts.
-
 Reactions in equilibrium
Reactions in equilibrium
Equilibrium reactions and constants.
-
 Mini-video on ion size
Mini-video on ion size
Correcting a mistake and learning a bit about ion size
-
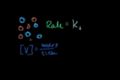 Keq intuition (mathy and not necessary to progress)
Keq intuition (mathy and not necessary to progress)
A probabilistic look at how molecules react to develop the intuition behind the equilibrium constant formula.
-
 Keq derivation intuition (can skip; bit mathy)
Keq derivation intuition (can skip; bit mathy)
A more concrete attempt at showing how the probabilities of molecules reacting is related to their concentration.
-
 Heterogeneous equilibrium
Heterogeneous equilibrium
Ignoring the solution or the solid state molecules when calculating the equilibrium constant.
-
 Le Chatelier's principle
Le Chatelier's principle
Le Chatelier's Principle regarding the "stressing" of reactions in equilibrium
-
 Introduction to pH, pOH, and pKw
Introduction to pH, pOH, and pKw
Autoionization of water into hydronium and hydroxide ions. pH, pOH, and pKa.