Khan Academy on a Stick
Chemical reactions (stoichiometry)
We are now going to delve into the heart of chemistry. We learn way of representing chemical molecules and how they react. To do this, we'll even think about "how many" of a molecule we have using a quantity called a "mole".
-
 Molecular and empirical formulas
Molecular and empirical formulas
Introduction to molecular and empirical formulas. Calculating molecular mass.
-
 The mole and avogadro's number
The mole and avogadro's number
Introduction to the idea of a mole as a number (vs. an animal).
-
 Empirical formula from mass composition
Empirical formula from mass composition
-
 Another mass composition problem
Another mass composition problem
Another exercise converting a mass composition to an empirical formula.
Molecular and empirical formulas
We'll now explore two different ways of representing what elements are in a molecule: molecular and empirical formulas. Molecular formulas actually represent the structure of a molecule while empirical formulas show us the ratio of the constituents based on experiments. In order to help us connect these ideas, we'll also explore a quantity called the "mole". Just as a "dozen" represents 12 of something, a "mole" represents roughly 602,200,000,000,000,000,000,000 of something.
Balancing chemical equations
We are now going to look at chemical reactions. But as we do, we need to make sure that atoms aren't magically appearing or disappearing. Put another way, we need to sure that we have the same number of each constituent atom in the product of the reaction as we do in the reactants (the molecules that react)!
-
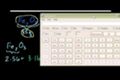 Stoichiometry
Stoichiometry
Introduction to stoichiometry.
-
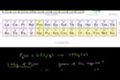 Stoichiometry example problem 1
Stoichiometry example problem 1
Figuring grams of reactants and product produced from reaction of phosphorous and chlorine.
-
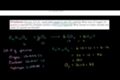 Stoichiometry example problem 2
Stoichiometry example problem 2
Stoichiometry
Now we are going to draw the connections between balancing equations and what happens in the lab (where you actually have a certain mass of a compound).
-
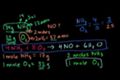 Stoichiometry: Limiting reagent
Stoichiometry: Limiting reagent
Stoichiometry problem where we have a limiting reagent!
-
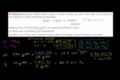 Limiting reactant example problem 1
Limiting reactant example problem 1
Limiting Reactant Example Problem 1
Limiting reagent stoichiometry
In a reaction, you often have extra of one molecule (or too little of the other) so all the reactant doesn't react. We'll explore how to identify which reactant is limiting which is helpful in a whole series of scenarios.
-
 Spectrophotometry introduction
Spectrophotometry introduction
Spectrophotometry, Transmittance, Absorbance and the Beer-Lambert Law
-
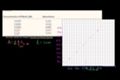 Spectrophotometry example
Spectrophotometry example
Spectrophotometry Example - Determining concentration based on absorbance
Spectrophotometry
In the lab, it is useful to know how much of something you have or the concentration of a solute. In this tutorial, we'll light to do that!
 Balancing chemical equations
Balancing chemical equations Balancing more complex chemical equation
Balancing more complex chemical equation Visually understanding balancing chemical equations
Visually understanding balancing chemical equations Balancing another combustion reaction
Balancing another combustion reaction Balancing chemical equation with substitution
Balancing chemical equation with substitution