Khan Academy on a Stick
Ideal gas laws
-
 Ideal gas equation: PV = nRT
Ideal gas equation: PV = nRT
Intuition behind the ideal gas equation: PV=nRT.
-
 Ideal gas equation example 1
Ideal gas equation example 1
Figuring out the number of moles of gas we have using the ideal gas equation: PV=nRT.
-
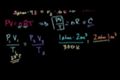 Ideal gas equation example 2
Ideal gas equation example 2
PV/T is a constant. Figuring out the volume of an ideal gas at standard temperature and pressure (STP).
-
 Ideal gas equation example 3
Ideal gas equation example 3
Figuring out the mass of Oxygen we have.
-
 Ideal gas equation example 4
Ideal gas equation example 4
Figuring out the molar mass of a mystery molecule at STP.
-
 Partial pressure
Partial pressure
Figuring out the partial pressures of various gases in a container.
-
 Vapor pressure example
Vapor pressure example
Vapor pressure example using the Ideal Gas Law