Tooth enamel
Background to the schools Wikipedia
SOS Children, an education charity, organised this selection. Sponsor a child to make a real difference.
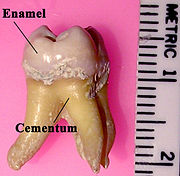
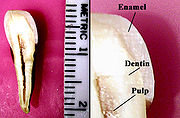
Tooth enamel is the hardest and most highly mineralized substance of the body, and with dentin, cementum, and dental pulp is one of the four major tissues which make up the tooth. It is the normally visible dental tissue of a tooth and must be supported by underlying dentin. Ninety-six percent of enamel consists of mineral, with water and organic material composing the rest. The normal color of enamel varies from light yellow to grayish white. At the edges of teeth where there is no dentin underlying the enamel, the colour sometimes has a slightly blue tone. Since enamel is semitranslucent, the colour of dentin and any restorative dental material underneath the enamel strongly affects the appearance of a tooth. Enamel varies in thickness over the surface of the tooth and is often thickest at the cusp, up to 2.5 mm, and thinnest at its border, which is seen clinically as the cementoenamel junction (CEJ).
Enamel's primary mineral is hydroxylapatite, which is a crystalline calcium phosphate. The large amount of minerals in enamel accounts not only for its strength but also for its brittleness. Tooth enamel is the hardest substance in the human body, ranking a 5 on Mohs hardness scale. Dentin, less mineralized and less brittle, 3-4 in hardness, compensates for enamel and is necessary as a support.
Unlike dentin and bone, enamel does not contain collagen. Instead, it has two unique classes of proteins called amelogenins and enamelins. While the role of these proteins is not fully understood, it is believed that they aid in the development of enamel by serving as a framework support, among other functions.
Structure
The basic unit of enamel is called an enamel rod. Measuring 4 μm - 8 μm in diameter an enamel rod, formerly called an enamel prism, is a tightly packed mass of hydroxyapatite crystals in an organized pattern. In cross section, it is best compared to a keyhole, with the top, or head, oriented toward the crown of the tooth, and the bottom, or tail, oriented toward the root of the tooth.
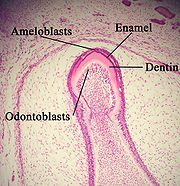
The arrangement of the crystals within each enamel rod is highly complex. Both ameloblasts (the cells which initiate enamel formation) and Tomes' processes affect the crystals' pattern. Enamel crystals in the head of the enamel rod are oriented parallel to the long axis of the rod. When found in the tail of the enamel rod, the crystals' orientation diverges slightly from the long axis.
The arrangement of enamel rods is understood more clearly than their internal structure. Enamel rods are found in rows along the tooth, and within each row, the long axis of the enamel rod is generally perpendicular to the underlying dentin. In permanent teeth, the enamel rods near the cementoenamel junction (CEJ) tilt slightly toward the root of the tooth. Understanding enamel orientation is very important in restorative dentistry, because enamel unsupported by underlying dentin is prone to fracture.
The area around the enamel rod is known as interrod enamel. Interrod enamel has the same composition as enamel rod, however a histologic distinction is made between the two because crystal orientation is different in each. The border where the crystals of enamel rods and crystals of interrod enamel meet is called the rod sheath.
Striae of Retzius are stripes that appear on enamel when viewed microscopically in cross section. Formed from changes in diameter of Tomes’ processes, these stripes demonstrate the growth of enamel, similar to the annual rings on a tree. Perikymata are shallow furrows where the striae of Retzius end. Darker than the other stripes, the neonatal line is a stripe that separates enamel formed before and after birth.
Gnarled enamel is found at the cusps of teeth. Its twisted appearance results from the orientation of enamel rods and the rows in which they lie.
Development
Enamel formation is part of the overall process of tooth development. When the tissues of the developing tooth are seen under a microscope, different cellular aggregations can be identified, including structures known as the enamel organ, dental lamina, and dental papilla. The generally recognized stages of tooth development are the bud stage, cap stage, bell stage, and crown, or calcification, stage. Enamel formation is first seen in the crown stage.
Amelogenesis, or enamel formation, occurs after the first establishment of dentin, via cells known as ameloblasts. Human enamel forms at a rate of around 4 μm per day, beginning at the future location of cusps, around the third or fourth month of pregnancy. As in all human processes, the creation of enamel is complex, but can generally be divided into two stages. The first stage, called the secretory stage, involves proteins and an organic matrix forming a partially mineralized enamel. The second stage, called the maturation stage, completes enamel mineralization.
In the secretory stage, ameloblasts are polarized columnar cells. In the rough endoplasmic reticulum of these cells, enamel proteins are released into the surrounding area and contribute to what is known as the enamel matrix, which is then partially mineralized by the enzyme alkaline phosphatase. When this first layer is formed, the ameloblasts move away from the dentin, allowing for the development of Tomes’ processes at the apical pole of the cell. Enamel formation continues around the adjoining ameloblasts, resulting in a walled area, or pit, that houses a Tomes’ process, and also around the end of each Tomes’ process, resulting in a deposition of enamel matrix inside of each pit. The matrix within the pit will eventually become an enamel rod, and the walls will eventually become interrod enamel. The only distinguishing factor between the two is the orientation of the calcium phosphate crystals.
In the maturation stage, the ameloblasts transport substances used in the formation of enamel. Histologically, the most notable aspect of this phase is that these cells become striated, or have a ruffled border. These signs demonstrate that the ameloblasts have changed their function from production, as in the secretory stage, to transportation. Proteins used for the final mineralization process compose most of the transported material. The noteworthy proteins involved are amelogenins, ameloblastins, enamelins, and tuftelins. During this process, amelogenins and ameloblastins are removed after use, leaving enamelins and tuftelin in the enamel. By the end of this stage, the enamel has completed its mineralization.
At some point before the tooth erupts into the mouth, but after the maturation stage, the ameloblasts are broken down. Consequently, enamel, unlike many other tissues of the body, has no way to regenerate itself. After destruction of enamel from decay or injury, neither the body nor a dentist can restore the enamel tissue. Enamel can be affected further by non-pathologic processes. The discoloration of teeth over time can result from exposure to substances such as tobacco, coffee, and tea. This is partly due to material building up in the enamel, but is also an effect of the underlying dentin becoming sclerotic. As a result, tooth colour gradually darkens with age. Additionally, enamel becomes less permeable to fluids, less soluble to acid, and contains less water.
| Amount of Enamel Formed at Birth | Enamel Mineralization Completed | ||
|---|---|---|---|
| Primary Maxillary Tooth |
Central Incisor | 5/6 | 1.5 months after birth |
| Lateral Incisor | 2/3 | 2.5 months after birth | |
| Canine | 1/3 | 9 months after birth | |
| 1st Molar | Cusps united; occlusal completely calcified and 1/2 to 3/4 crown height |
6 months after birth | |
| 2nd Molar | Cusps united; occlusal incompletely calcified; calcified tissue covers 1/5 to 1⁄4 crown height |
11 months after birth | |
| Primary Mandibular Tooth |
Central Incisor | 3/5 | 2.5 months after birth |
| Lateral Incisor | 3/5 | 3 months after birth | |
| Canine | 1/3 | 9 months after birth | |
| 1st Molar | Cusps united; occlusal completely calcified |
5.5 months after birth | |
| 2nd Molar | Cusps united; occlusal incompletely calcified |
10 months after birth | |
Destruction
The high mineral content of enamel, which makes this tissue the hardest in the human body, also makes it susceptible to a demineralization process which often occurs as dental caries, otherwise known as cavities. Demineralization occurs for several reasons, but the most important cause of tooth decay is the ingestion of sugars. Tooth cavities are caused when acids dissolve tooth enamel:
-
- Ca10(PO4)6(OH)2(s) + 8H+(aq) → 10Ca2+(aq) + 6HPO42-(aq) + 2H2O(l)
Sugars from candies, soft drinks, and even fruit juices play a significant role in tooth decay, and consequently in enamel destruction. The mouth contains a great number and variety of bacteria, and when sucrose, the most common of sugars, coats the surface of the mouth, some intraoral bacteria interact with it and form lactic acid, which decreases the pH in the mouth. Then, the hydroxylapatite crystals of enamel demineralize, allowing for greater bacterial invasion deeper into the tooth. The most important bacterium involved with tooth decay is Streptococcus mutans, but the number and type of bacteria varies with the progress of tooth destruction.
Furthermore, tooth morphology dictates that the most common site for the initiation of dental caries is in the deep grooves, pits, and fissures of enamel. This is expected because these locations are impossible to reach with a toothbrush and allow for bacteria to reside there. When demineralization of enamel occurs, a dentist can use a sharp instrument, such as a dental explorer, and "feel a stick" at the location of the decay. As enamel continues to become less mineralized and is unable to prevent the encroachment of bacteria, the underlying dentin becomes affected as well. When dentin, which normally supports enamel, is destroyed by a physiologic condition or by decay, enamel is unable to compensate for its brittleness and breaks away from the tooth easily.
The extent to which tooth decay is likely, known as cariogenicity, depends on factors such as how long the sugar remains in the mouth. Contrary to common belief, it is not the amount of sugar ingested but the frequency of sugar ingestion that is the most important factor in the causation of tooth decay. When the pH in the mouth initially decreases from the ingestion of sugars, the enamel is demineralized and left vulnerable for about 30 minutes. Eating a greater quantity of sugar in one sitting does not increase the time of demineralization. Similarly, eating a lesser quantity of sugar in one sitting does not decrease the time of demineralization. Thus, eating a great quantity of sugar at one time in the day is less detrimental than is a very small quantity ingested in many intervals throughout the day. For example, in terms of oral health, it is better to eat a single dessert at dinner time than to snack on a bag of candy throughout the day.
In addition to bacterial invasion, enamel is also susceptible to other destructive forces. Bruxism, also known as clenching of or grinding on teeth, destroys enamel very quickly. The wear rate of enamel, called attrition, is 8 micrometers a year from normal factors. A common misconception is that enamel wears away mostly from chewing, but actually teeth rarely touch during chewing. Furthermore, normal tooth contact is compensated physiologically by the periodontal ligaments (pdl) and the arrangement of dental occlusion. The truly destructive forces are the parafunctional movements, as found in bruxism, which can cause irreversible damage to the enamel.
Other nonbacterial processes of enamel destruction include abrasion (involving foreign elements, such as toothbrushes), erosion (involving chemical processes, such as lemon juice), and possibly abfraction (involving compressive and tensile forces).
Oral hygiene and fluoride
Considering the vulnerability of enamel to demineralization and the daily menace of sugar ingestion, prevention of tooth decay is the best way to maintain the health of teeth. Most countries have wide use of toothbrushes, which can reduce the number of bacteria and food particles on enamel. Some isolated societies do not have access to toothbrushes, but it is common for those people to use other objects, such as sticks, to clean their teeth. In between two adjacent teeth, floss is used to wipe the enamel surfaces free of plaque and food particles to discourage bacterial growth. Although neither floss nor toothbrushes can penetrate the deep grooves and pits of enamel, good general oral health habits can usually prevent enough bacterial growth to keep tooth decay from starting.
These methods of oral hygiene have been helped greatly by the use of fluoride. Fluoride can be found in many locations naturally, such as the ocean and other water sources. Consequently, many seafood dishes contain fluoride. The recommended dosage of fluoride in drinking water is 1 part per million ( ppm). Fluoride helps prevent dental decay by binding to the hydroxylapatite crystals in enamel. The incorporated fluoride makes enamel more resistant to demineralization and, thus, resistant to decay. Fluoride therapy is used to help teeth prevent dental decay.
Many groups of people have spoken out against fluoridated drinking water. One example used by these advocates is the damage fluoride can do as fluorosis. Fluorosis is a condition resulting from the overexposure to fluoride, especially between the ages of 6 months to 5 years, and appears as mottled enamel. Consequently, the teeth look unsightly and, indeed, the incidence of dental decay in those teeth is very small. However, it is important to note that most substances, even beneficial ones, are detrimental when taken in extreme doses. Where fluoride is found naturally in high concentrations, filters are often used to decrease the amount of fluoride in water. For this reason, codes have been developed by dental professionals to limit the amount of fluoride a person should take. These codes are supported by the American Dental Association and the American Academy of Pediatric Dentistry. The acute toxic dose of fluoride is ~5 mg/kg of body weight. Furthermore, whereas topical fluoride, found in toothpaste and mouthwashes, does not cause fluorosis, its effects are also less pervasive and not as long-lasting as those of systemic fluoride, such as when drinking fluorinated water. For instance, all of a tooth's enamel gains the benefits of fluoride when it is ingested systemically, through fluoridated water or salt fluoridation (a common alternative in Europe). Only some of the outer surfaces of enamel can be reached by topical fluoride. Thus, despite fluoridation's detractors, most dental health care professionals and organizations agree that the inclusion of fluoride in public water has been one of the most effective methods of decreasing the prevalence of tooth decay.
Effects of dental procedures
Dental restorations
Most dental restorations involve the removal of enamel. Frequently, the purpose of removal is to gain access to the underlying decay in the dentin or inflammation in the pulp. This is typically the case in amalgam restorations and endodontic treatment.
Nonetheless, enamel can sometimes be removed before there is any decay present. The most popular example is the dental sealant. The process of placing dental sealants in the past involved removing enamel in the deep fissures and grooves of a tooth and replacing it with a restorative material. Presently, it is more common to only remove decayed enamel if present. In spite of this, there are still cases where deep fissures and grooves in enamel are removed in order to prevent decay, and a sealant may or may not be placed depending on the situation. Sealants are unique in that they are preventative restorations for protection from future decay and have shown to reduce the risk of decay by 55% over 7 years.
Aesthetics is another reason for the removal of enamel. Removing enamel is necessary when placing crowns and veneers to enhance the appearance of teeth. In both of these instances, it is important to keep in mind the orientation of enamel rods because it is possible to leave enamel unsupported by underlying dentin, leaving that portion of the prepared teeth more vulnerable to fracture.
Acid-etching techniques
Invented in 1955, acid-etching employs dental etchants and is used frequently when bonding dental restoration to teeth. This is important for long-term use of some materials, such as composites and sealants. By dissolving minerals in enamel, etchants remove the outer 10 micrometers on the enamel surface and makes a porous layer 5–50 micrometers deep. This roughens the enamel microscopically and results in a greater surface area on which to bond.
The effects of acid-etching on enamel can vary. Important variables are the amount of time the etchant is applied, the type of etchant used, and the current condition of the enamel.
There are three types of patterns formed by acid-etching. Type 1 is a pattern where predominantly the enamel rods are dissolved; type 2 is a pattern where predominantly the area around the enamel rods are dissolved; and type 3 is a pattern where there is no evidence left of any enamel rods. Besides concluding that type 1 is the most favorable pattern and type 3 the least, the explanation for these different patterns is not known for certain but is most commonly attributed to different crystal orientation in the enamel.
Tooth whitening
Tooth whitening or tooth bleaching are procedures that attempt to lighten a tooth's colour in either of two ways: by chemical or mechanical action.
Working chemically, a bleaching agent is used to carry out an oxidation reaction in the enamel and dentin. The agents most commonly used to intrinsically change the colour of teeth are hydrogen peroxide and carbamide peroxide. A tooth whitening product with an overall low pH can put enamel at risk for decay or destruction by demineralization. Consequently, care should be taken and risk evaluated when choosing a product which is very acidic.
Tooth whiteners in toothpastes work through a mechanical action. They have mild abrasives which aid in the removal of stains on enamel. Although this can be an effective method, it does not alter the intrinsic colour of teeth.
Microabrasion techniques employ both methods. An acid is used first to weaken the outer 22–27 micrometers of enamel in order to weaken it enough for the subsequent abrasive force. This allows for removal of superficial stains in the enamel. If the discoloration is deeper or in the dentin, this method of tooth whitening will not be successful.
Systemic conditions affecting enamel
There are many different types of Amelogenesis imperfecta. The hypocalcification type, which is the most common, is an autosomal dominant condition that results in enamel that is not completely mineralized. Consequently, enamel easily flakes off the teeth, which appear yellow because of the revealed dentin. The hypoplastic type is X-linked and results in normal enamel that appears in too little quantity, having the same effect as the most common type.
Chronic bilirubin encephalopathy, which can result from erythroblastosis fetalis, is a disease which has numerous effects on an infant, but it can also cause enamel hypoplasia and green staining of enamel.
Enamel hypoplasia is broadly defined to encompass all deviations from normal enamel in its various degrees of absence. The missing enamel could be localized, forming a small pit, or it could be completely absent.
Erythropoietic porphyria is a genetic disease resulting in the deposition of porphyrins throughout the body. These deposits also occur in enamel and leave an appearance described as red in colour and fluorescent.
Fluorosis leads to mottled enamel and occurs from overexposure to fluoride.
Tetracycline staining leads to brown bands on the areas of developing enamel. Children up to age 8 can develop mottled enamel from taking tetracycline. As a result, tetracycline is contraindicated in pregnant women.
Celiac disease, an auto-immune disorder triggered by gluten allergies, also commonly results in demineralization of the enamel.
Enamel in animals
For the most part, research has shown that formation in animals is almost identical to formation in humans. The enamel organ, including the dental papilla, and ameloblasts function similarly. The variations of enamel that are present are infrequent but sometimes important. Differences exist, certainly, in the morphology, number, and types of teeth among animals.
Dogs are less likely than humans to have tooth decay due to the high pH of dog saliva, which prevents an acidic environment from forming and the subsequent demineralization of enamel which would occur. In the event that tooth decay does occur (usually from trauma), dogs can receive dental fillings just as humans do. Similar to human teeth, the enamel of dogs is vulnerable to tetracycline staining. Consequently, this risk must be accounted for when tetracycline antibiotic therapy is administered to young dogs. Enamel hypoplasia may also occur in dogs.
The mineral distribution in rodent enamel is different from that of monkeys, dogs, pigs, and humans. In horse teeth, the enamel and dentin layers are intertwined with each other, which increases the strength and decreases the wear rate of those teeth.