Gold(III) chloride
Did you know...
SOS Children made this Wikipedia selection alongside other schools resources. Before you decide about sponsoring a child, why not learn about different sponsorship charities first?
| Gold(III) chloride | |
|---|---|
 |
|
 |
|
 |
|
|
Gold(III) chloride |
|
|
Other names
Auric chloride |
|
| Identifiers | |
| CAS number | 13453-07-1 |
| PubChem | 26030 |
| ChemSpider | 8036939 |
| UNII | 15443PR153 |
| ChEBI | CHEBI:30076 |
| RTECS number | MD5420000 |
| Jmol-3D images | Image 1 |
|
SMILES
|
|
|
InChI
|
|
| Properties | |
| Molecular formula | AuCl3 (exists as Au2Cl6) |
| Molar mass | 303.325 g/mol |
| Appearance | Red crystals (anhydrous); golden, yellow crystals (monohydrate) |
| Density | 4.7 g/cm3 |
| Melting point |
254 °C (527 K) |
| Solubility in water | 68 g/100 ml (cold) |
| Solubility | soluble in ether, slightly soluble in liquid ammonia |
| Structure | |
| Crystal structure | monoclinic |
| Coordination geometry |
Square planar |
| Hazards | |
| MSDS | External MSDS |
| R-phrases | R36/37/38 |
| S-phrases | S26 S36 |
| Main hazards | Irritant |
| Related compounds | |
| Other anions | Gold(III) fluoride Gold(III) bromide |
| Other cations | Gold(I) chloride Silver(I) chloride Platinum(II) chloride Mercury(II) chloride |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
| Infobox references | |
Gold(III) chloride, traditionally called auric chloride, is a chemical compound of gold and chlorine. With the molecular formula Au2 Cl6, the name gold trichloride is a simplification, referring to the empirical formula. The Roman numerals in the name indicate that the gold has an oxidation state of +3, which is common for gold compounds. There is also another related chloride of gold, gold(I) chloride (AuCl). Chloroauric acid, HAuCl4, the product formed when gold dissolves in aqua regia, is sometimes referred to as "gold chloride", "acid gold trichloride" or even "gold(III) chloride trihydrate." Gold(III) chloride is very hygroscopic and highly soluble in water as well as ethanol. It decomposes above 160 °C or in light.
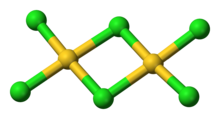
Structure
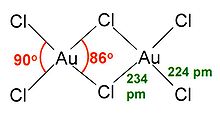
AuCl3 exists as a chloride-bridged dimer both as a solid and as a vapour, at least at low temperatures. Gold(III) bromide behaves analogously. The structure is similar to that of iodine(III) chloride.
In gold(III) chloride, each gold centre is square planar, which is typical of a metal complex with a d8 electronic count. The bonding in AuCl3 is considered somewhat covalent.
Preparation
Gold(III) chloride is most often prepared by passing chlorine gas over gold powder at 180 °C:
- 2 Au + 3 Cl2 → 2 AuCl3
Another method of preparation, is the addition reaction, where gold(3+) is reacted with chloride to produce tetrachloridoaurate(1-); after which, the hydrogen tetrachloridoaurate(1-) is heated to eliminate chlorane gas. Gold(3+) is produced by oxidising with nitric acid present in aqua regia:
- Au(s) + 3 NO−
3(aq) + 6 H+(aq) Au3+(aq) + 3 NO2(g) + 3 H2O(l)
Au3+(aq) + 3 NO2(g) + 3 H2O(l) - Au3+(aq) + 3 NOCl(g) + 3 NO−
3(aq) → AuCl3(aq) + 6 NO2(g) - AuCl3(aq) + Cl-(aq)
 AuCl−
AuCl−
4(aq) - 2 HAuCl4(s) → Au2Cl6(s) + 2 HCl(g)
Reactions
On contact with water, AuCl3 forms a series of species, sometimes described as AuCl3·H2O and its conjugate base [AuCl3(OH)]−. Reaction with reducing agents such as hydrogen peroxide or Fe2+ causes elemental gold to be precipitated from solution.
Anhydrous AuCl3 begins to decompose to AuCl at around 160 °C; however, this in turn undergoes disproportionation at higher temperatures to give gold metal and AuCl3.
- AuCl3 → AuCl + Cl2 (>160 °C)
- 3 AuCl → AuCl3 + 2 Au (>420 °C)
AuCl3 is Lewis acidic and readily forms complexes. For example, it reacts with hydrochloric acid to form chloroauric acid (HAuCl4):
- HCl + AuCl3 (aq) → H+ + [AuCl4]−
Other chloride sources, such as KCl, also convert AuCl3 into AuCl−
4. Aqueous solutions of AuCl3 react with aqueous base such as sodium hydroxide to form a precipitate of Au(OH)3, which will dissolve in excess NaOH to form sodium aurate (NaAuO2). If gently heated, Au(OH)3 decomposes to gold(III) oxide, Au2O3, and then to gold metal.
Gold(III) chloride is the starting point for the synthesis of many other gold compounds. For example, reaction with potassium cyanide produces the water-soluble complex, K[Au(CN)4]:
- AuCl3 + 4 KCN → K[Au(CN)4] + 3 KCl
Applications in organic synthesis
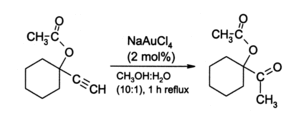
AuCl3 has attracted the interest of organic chemists as a mild acid catalyst for a variety reactions, although no transformations have been commercialized. Gold(III) salts, especially Na[AuCl4] (prepared from AuCl3 + NaCl), provide an alternative to mercury(II) salts as catalysts for reactions involving alkynes. An illustrative reaction is the hydration of terminal alkynes to produce methylketones:
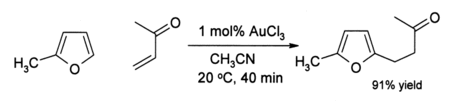
Some alkynes undergo amination in the presence of gold(III) catalysts. Gold catalyses the alkylation of certain aromatic rings and a conversion of furans to phenols. For example, in acetonitrile solution, gold(III) chloride catalyses the alkylation of 2-methylfuran (sylvan) by methyl vinyl ketone at the 5-position:
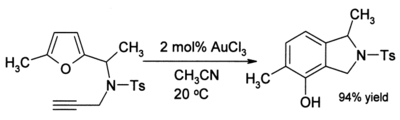
The efficiency of this organogold reaction is noteworthy because both the furan and the ketone are sensitive to side-reactions such as polymerisation under acidic conditions. In some cases where alkynes are present, phenols sometimes form:
This reaction involves a rearrangement that gives a new aromatic ring.