Acid–base reaction
Did you know...
The articles in this Schools selection have been arranged by curriculum topic thanks to SOS Children volunteers. Sponsor a child to make a real difference.
| Acids and Bases |
|---|
| Acid dissociation constant Acid-base extraction Acid–base reaction Acid–base titration Dissociation constant Acidity function Buffer solutions pH Proton affinity Self-ionization of water Acid strength |
| Acid types |
| Brønsted · Lewis · Mineral Organic · Strong Superacids · Weak |
| Base types |
| Brønsted · Lewis · Organic Strong · Superbases Non-nucleophilic · Weak |
An acid-base reaction is a chemical reaction that occurs between an acid and a base. Several concepts exist which provide alternative definitions for the reaction mechanisms involved and their application in solving related problems. Despite several similarities in definitions, their importance becomes apparent as different methods of analysis when applied to acid-base reactions for gaseous or liquid species, or when acid or base character may be somewhat less apparent. Historically, the first of these scientific concepts of acids and bases was provided by the French chemist Antoine Lavoisier, circa 1776.
Common acid-base theories
Lavoisier definition
Since Lavoisier's knowledge of strong acids was mainly restricted to oxyacids, which tend to contain central atoms in high oxidation states surrounded by oxygen, such as HNO3 and H2SO4, and since he was not aware of the true composition of the hydrohalic acids, HCl, HBr, and HI, he defined acids in terms of their containing oxygen, which in fact he named from Greek words meaning "acid-former" (from the Greek οξυς (oxys) meaning "acid" or "sharp" and γεινομαι (geinomai) or "engender"). The Lavoisier definition was held as absolute truth for over 30 years, until the 1810 article and subsequent lectures by Sir Humphry Davy in which he proved the lack of oxygen in H2S, H2Te, and the hydrohalic acids.
Liebig definition
This definition was proposed by Justus von Liebig circa 1838, based on his extensive works on the chemical composition of organic acids. This finished the doctrinal shift from oxygen-based acids to hydrogen-based acids, started by Davy. According to Liebig, an acid is a hydrogen-containing substance in which the hydrogen could be replaced by a metal. Liebig's definition, while completely empirical, remained in use for almost 50 years until the adoption of the Arrhenius definition.
Arrhenius definition
The Arrhenius definition of acid-base reactions is a more simplified acid-base concept devised by Svante Arrhenius, which was used to provide a modern definition of bases that followed from his work with Friedrich Wilhelm Ostwald in establishing the presence of ions in aqueous solution in 1884, and led to Arrhenius receiving the Nobel prize in chemistry in 1903 for "recognition of the extraordinary services ... rendered to the advancement of chemistry by his electrolytic theory of dissociation"
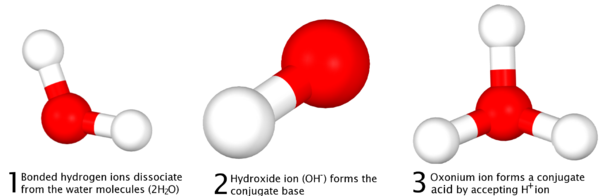
As defined at the time of discovery, acid-base reactions are characterized by Arrhenius acids, which dissociate in aqueous solution form hydrogen or the later-termed oxonium (H3O+) ions, and Arrhenius bases which form hydroxide (OH-) ions. More recent IUPAC recommendations now suggest the newer term "hydronium" be used in favour of the older accepted term "oxonium" to illustrate reaction mechanisms such as those defined in the Brønsted-Lowry and solvent system definitions more clearly, with the Arrhenius definition serving as a simple general outline of acid-base character More succinctly, the Arrhenius definition can be surmised as;
| “ | Arrhenius acids form hydrogen ions in aqueous solution with Arrhenius bases forming hydroxide ions. | ” |
The universal aqueous acid-base definition of the Arrhenius concept is described as the formation of water from hydrogen and hydroxide ions, or hydronium ions and hydroxide ions produced from the dissociation of an acid and base in aqueous solution (2 H2O → OH- + H3O+ ), which leads to the definition that in Arrhenius acid-base reactions, a salt and water is formed from the reaction between an acid and a base -- in more simple scientific definitions, this form of reaction is called a Neutralization reaction.
- acid+ + base- → salt + water
The positive ion from a base can form a salt with the negative ion from an acid. For example, two moles of the base sodium hydroxide (NaOH) can combine with one mole of sulfuric acid (H2SO4) to form two moles of water and one mole of sodium sulfate.
- 2NaOH + H2SO4 → 2 H2O + Na2SO4
Brønsted-Lowry definition
The Brønsted-Lowry definition, formulated independently by its two proponents Johannes Nicolaus Brønsted and Martin Lowry in 1923 is based upon the idea of protonation of bases through the de-protonation of acids -- more commonly referred to as the ability of acids to "donate" hydrogen ions (H+) or protons to bases, which "accept" them. In contrast to the Arrhenius definition, the Brønsted-Lowry definition refers to the products of an acid-base reaction as conjugate acids and bases to refer to the relation of one proton, and to indicate that there has been a reaction between the two quantities, rather than a "formation" of salt and water, as explained in the Arrhenius definition.
It defines that in reactions, there is the donation and reception of a proton, which essentially refers to the removal of a hydrogen ion bonded within a compound and its reaction with another compound, and not the removal of a proton from the nucleus of an atom, which would require inordinate amounts of energy not attainable through the simple dissociation of acids. In differentiation from the Arrhenius definition, the Brønsted-Lowry definition postulates that for each acid, there is a conjugate acid and base or "conjugate acid-base pair" that is formed through a complete reaction, which also includes water, which is amphoteric:
|
Lewis definition
The Lewis definition of acid base reactions, devised by Gilbert N. Lewis in 1923 is an encompassing theory to the Brønsted-Lowry and solvent-system definitions with regards to the premise of a donation mechanism, which conversely attributes the donation of electron pairs from bases and the acceptance by acids, rather than protons or other bonded substances and spans both aqueous and non-aqueous reactions.
- Ag+ + 2 :NH3 → [H3N:Ag:NH3]+
- A silver cation reacts as an acid with ammonia which acts as an electron-pair donor, forming an ammonia-silver adduct
In reactions between Lewis acids and bases, there is the formation of an adduct when the highest occupied molecular orbital ( HOMO) of a molecule, such as NH3 with available lone electron pair(s) donates lone pairs of electrons to the electron-deficient molecule's lowest unoccupied molecular orbital ( LUMO) through a co-ordinate covalent bond; in such a reaction, the HOMO-interacting molecule acts as a base, and the LUMO-interacting molecule acts as an acid. In highly-polar molecules, such as Boron Tri-fluoride (BF3), the most electronegative element pulls electrons towards its own orbitals, providing a more positive charge on the less-electronegative element and a difference in its electronic structure due to the axial or equatorial orbiting positions of its electrons, causing repulsive effects from Lone pair-bonding pair (Lp-Bp) interactions between bonded atoms in excess of those already provided by Bonding pair-bonding pair (Bp-Bp) interactions. Adducts involving metal ions are referred to as co-ordination compounds.
Solvent-system definition
This definition is based on a generalization of the earlier Arrhenius definition to all autodissociating solvents. In all such solvents there is a certain concentration of a positive species, solvonium cations and negative species, solvate anions, in equilibrium with the neutral solvent molecules. For example:
- 2H2O ⇌ H3O+ ( hydronium) + OH- (hydroxide)
- 2NH3 ⇌ NH4+ ( ammonium) + NH2− ( amide)
or even some aprotic systems
- N2O4 ⇌ NO+ ( nitrosonium) + NO3− (nitrate)
- 2SbCl3 ⇌ SbCl2+ (dichloroantimonium) + SbCl4- (tetrachloroantimonate)
A solute causing an increase in the concentration of the solvonium ions and a decrease in the solvate ions is an acid and one causing the reverse is a base. Thus, in liquid ammonia, KNH2 (supplying NH2-) is a strong base, and NH4NO3 (supplying NH4+) is a strong acid. In liquid sulfur dioxide (SO2), thionyl compounds (supplying SO2+) behave as acids, and sulfites (supplying SO32−) behave as bases.
Here are some nonaqueous acid-base reactions in liquid ammonia
- 2NaNH2 (base) + Zn(NH2)2 ( amphiphilic amide) → Na2[Zn(NH2)4]
- 2NH4I (acid) + Zn(NH2)2 ( amphiphilic amide) → [Zn(NH3)4)]I2
Nitric acid can be a base in liquid sulfuric acid:
- HNO3 (base) + 2H2SO4 → NO2+ + H3O+ + 2HSO4-
And things become even stranger in the aprotic world, for example in liquid N2O4:
- AgNO3 (base) + NOCl (acid) → N2O4 + AgCl
Since solvent-system definition depends on the solvent as well as on the compound itself, the same compound can change its role depending on the choice of the solvent. Thus, HClO4 is a strong acid in water, a weak acid in acetic acid, and a weak base in fluorosulfonic acid.
Other acid-base theories
Usanovich definition
The most general definition is that of the Russian chemist Mikhail Usanovich, and can basically be summarized as defining an acid as anything that accepts negative species or donates positive ones, and a base as the reverse. This tends to overlap the concept of redox (oxidation-reduction), and so is not highly favored by chemists. This is because redox reactions focus more on physical electron transfer processes, rather than bond making/bond breaking processes, although the distinction between these two processes is somewhat ambiguous.
Lux-Flood definition
This definition, proposed by German chemist Hermann Lux in 1939, further improved by Håkon Flood circa 1947 and now commonly used in modern geochemistry and electrochemistry of molten salts, describes an acid as an oxide ion acceptor and a base as an oxide ion donor. For example:
- MgO (base) + CO2 (acid) → MgCO3
- CaO (base) + SiO2 (acid) → CaSiO3
- NO3- (base) + S2O72- (acid) → NO2+ + 2SO42-
Pearson definition
In 1963 Ralph Pearson proposed an advanced qualitative concept known as Hard Soft Acid Base principle, later made quantitative with help of Robert Parr in 1984. 'Hard' applies to species which are small, have high charge states, and are weakly polarizable. 'Soft' applies to species which are large, have low charge states and are strongly polarizable. Acids and bases interact and the most stable interactions are hard-hard and soft-soft. This theory has found use in both organic and inorganic chemistry.