
File:Close-packed spheres.jpg
| |
This is a file from the Wikimedia Commons. Information from its description page there is shown below.
Commons is a freely licensed media file repository. You can help. |
| This image was created with Ashlar-Vellum Cobalt. |
Summary
| DescriptionClose-packed spheres.jpg |
see below |
| Date | |
| Source | English Wikipedia |
| Author | User:Greg L |
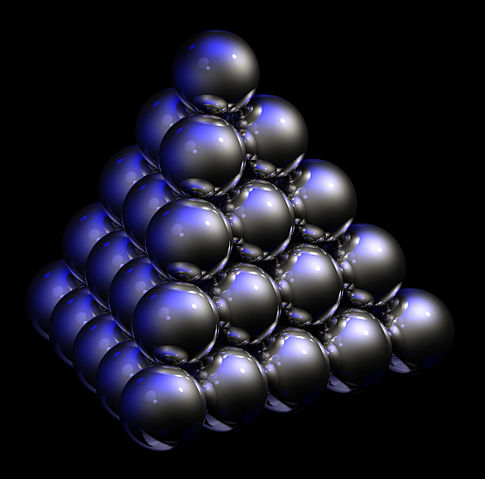
Shown above is what the science of sphere packing calls a closest-packed arrangement. Specifically, this is the cannonball arrangement or cannonball stack. Thomas Harriot in ca. 1585 first pondered the mathematics of cannonball stacks and later asked Johannes Kepler if the stack illustrated here was truly the most efficient. Kepler wrote, in what today is known as the Kepler conjecture, that no other arrangement of spheres can exceed its packing density of 74%.
Mathematically, there is an infinite quantity of closest-packed arrangements (assuming an infinite-size volume in which to arrange spheres). In the field of crystal structure however, unit cells (a crystal’s repeating pattern) are composed of a limited number of atoms and this reduces the variety of closest-packed regular lattices found in nature to only two: hexagonal close packed (HCP), and face-centered cubic (FCC). As can be seen at this site at King’s College, there is a distinct, real difference between different lattices; it’s not just a matter of how one slices 3D space. With all closest-packed lattices however, any given internal atom is in contact with 12 neighbors — the maximum possible.
Note that this stack is not a FCC unit cell since this group can not tessellate in 3D space. Visit the King’s College Web site to see HCP and FCC unit cells.
When many chemical elements (such as most of the noble gases and platinum-group metals) freeze solid, their lattice unit cells are of the FCC form. Having a closest-packed arrangement is one of the reasons why iridium and osmium (both of which are platinum-group metals) have the two greatest bulk densities of all the chemical elements.
The stack shown here is indeed quite dense. If this stack of 35 spheres was composed of iron cannonballs, each measuring 10 cm in diameter, the top of the stack would be only 42.66 cm off the ground — just under the knee of the average barefoot man — and yet would weigh over 144 kg.
- ↑ To 23 significant digits, the value is 74.048 048 969 306 104 116 931%
Rendered and modeled using Cobalt CAD package.
Licensing
|
upload from http://en.wikipedia.org/wiki/Image:Close-packed_spheres.jpg
File usage
Metadata
| Orientation | Normal |
|---|---|
| Horizontal resolution | 72 dpi |
| Vertical resolution | 72 dpi |
| Software used | Adobe Photoshop 7.0 |
| File change date and time | 09:01, 18 February 2007 |
| Colour space | Uncalibrated |
Schools Wikipedia facts
Schools Wikipedia was created by children's charity SOS Children's Villages. In 133 nations around the world, SOS Children works to bring better education and healthcare to families in desperate need of support. Sponsoring a child is the coolest way to help.

